In the ever-evolving landscape of medical device security, 2023 brings forth a nuanced set of challenges, reflecting the industry’s deeper understanding of the intricacies involved. This blog delves into the top challenges faced by Medical Device Manufacturers (MDMs) this year, shedding light on how the complexity of device security is unfolding.
To download our full 2023 Medical Device Security Survey Report, click here.
Key challenges
MDMs face complex issues that underscore the critical intersection of technology, processes, and organizational adaptation. In order to gain a better understanding of these challenges, we asked survey respondents what their top medical device security challenge was for 2023.
Frictionless product security processes with R&D
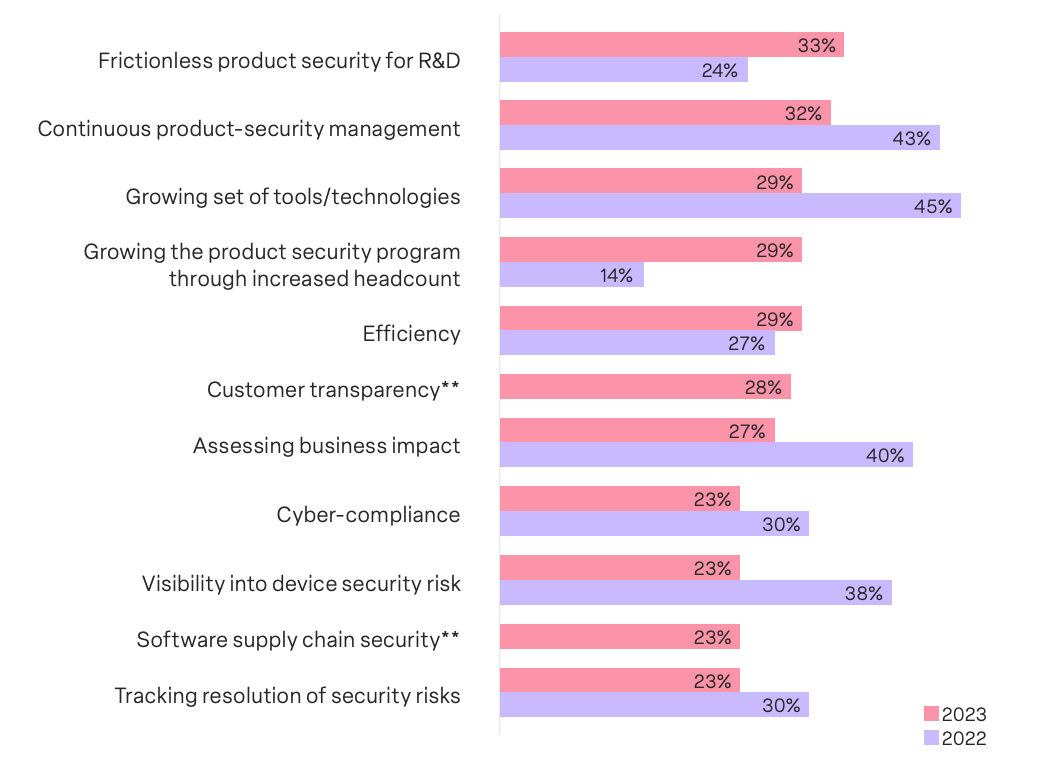
Ensuring frictionless product security processes with Research & Development (R&D) emerged as the top challenge, chosen by 33% of MDMs. Establishing harmonious collaboration between security teams and R&D is pivotal to embedding security measures at the core of the device development lifecycle. It is evident that MDMs understand the need to adopt a proactive approach where security is not an afterthought but an inherent aspect woven into the fabric of product development.
Continuous product security management
A close second to the top challenge was continuous product security management throughout a device’s lifespan, chosen by 32% of respondents. The high placement of this challenge indicates that MDMs recognize the need for a lifecycle-oriented security strategy that extends beyond initial development, something that is also mandated by regulations and guidance such as those set by the FDA. This strategy requires MDMs to adopt measures that ensure ongoing security enhancements, threat monitoring, and prompt response to emerging risks throughout a device’s lifespan.

Evolving landscape of tools and technologies
The third top medical device security challenge for 2023, chosen by 29% of respondents, is the growing set of tools and technologies used for product security. Staying abreast of the latest advancements, ensuring compatibility, and integrating new solutions seamlessly can all pose significant hurdles to MDMs. This challenge’s high placement accentuates the importance of adaptability, a proactive mindset, and strategic investments in technologies that fortify rather than impede the efficacy of medical device security measures.
Emerging challenges for 2023
As the landscape of medical device security continues to evolve, so do the challenges MDMs face. A comparative analysis with our 2022 survey sheds light on emerging concerns for 2023, indicating an overall maturation in MDM’s understanding of product security.
One of the noteworthy shifts is the ascent of streamlining Research and Development (R&D) processes, shifting from a low rank of 24% in 2022 to a top concern for 33% of respondents in 2023. This underscores a nuanced recognition among MDMs of the critical intersection between security and the developmental phases of medical devices.
Another key trend is the heightened emphasis on expanding security team headcount, climbing from a low rank of 14% in 2022 to a top concern for 29% in 2023. This shift signifies a practical acknowledgment of the escalating complexities in the security landscape. MDMs recognize the imperative to bolster their security teams to effectively address evolving threats and challenges.
These results make it evident that MDMs are maturing in their understanding of the intricate field of product security. However, they also now face practical challenges they may not have encountered before. As MDMs navigate these emerging challenges, a strategic and proactive approach to medical device security becomes paramount.
Discrepancies between suppliers and OEMs

While tracking the resolution of security risk emerged as the lowest challenge chosen by all respondents, a closer examination reveals intriguing divergences between suppliers and Original Equipment Manufacturers (OEMs). Suppliers assign significantly higher importance (31.8%) to tracking the resolution of security risks compared to their OEM counterparts (18.9%).
This finding implies a bottom-up challenge within the ecosystem. While OEMs may prioritize other aspects of their security strategies, suppliers underscore the criticality of vigilantly monitoring and resolving security issues. This nuanced insight urges a comprehensive understanding of the diverse perspectives within the medical device supply chain. Tailoring security approaches to accommodate the unique challenges faced by suppliers will contribute to a more robust and holistic security ecosystem.
The multitude of medical device security challenges
Notably, our survey reveals that MDMs grapple with a diverse array of concerns across various categories, with 11 different challenges chosen by 23% or more of survey respondents. This diversity in challenges, each holding a similar weightage, serves as a poignant reflection of the multifaceted nature of device security. From evolving tools and technologies to cyber-compliance and software supply chain security, MDMs navigate a terrain rife with diverse and interrelated challenges.With a striking 68% of companies’ security programs lacking the desired level of maturity, the simultaneous presence of myriad challenges is hardly surprising. It underscores the urgent need for improvement and a strategic overhaul of device security initiatives. The interplay of challenges serves as a compelling call for organizations to address the root causes, enhance program maturity, and fortify their defenses against the evolving threats in the medical device landscape.
Navigating forward in the realm of medical device security
In uncovering the top challenges for Medical Device Manufacturers this year, it’s clear that the industry is evolving in its approach to security. Yet, with diverse challenges and a significant percentage of programs lacking maturity, the urgency for strategic improvements resonates. The interplay of these challenges calls for comprehensive solutions to fortify device security in a landscape of constant change.
Uncover our in-depth analysis of the top challenges shaping the landscape of medical device security in 2023. Download the full Medical Device Security Survey Report for a comprehensive understanding of the industry’s current state and the path forward.
Download the full 2024 Medical Device Security Survey Report