The information and data below are taken from our 2023 Medical Device Security Survey Report. To view the full report, click here.
In the ever-evolving landscape of medical device security, Medical Device Manufacturers (MDMs) are putting their efforts under a magnifying glass. As such, the practice of meeting the compliance minimums dropped significantly from 78% of respondents in 2022 to only 26% in 2023. What’s more, improving compliance submission success rates has become the number two priority for 2023 Product Security roadmaps.
Our survey brings forth a compelling narrative of the surge in commitment displayed by MDMs toward adhering to regulations, standards, and guidelines. This blog delves deep into the findings, unpacking the key trends and nuances that shape the compliance landscape in 2023.
Rising compliance rates
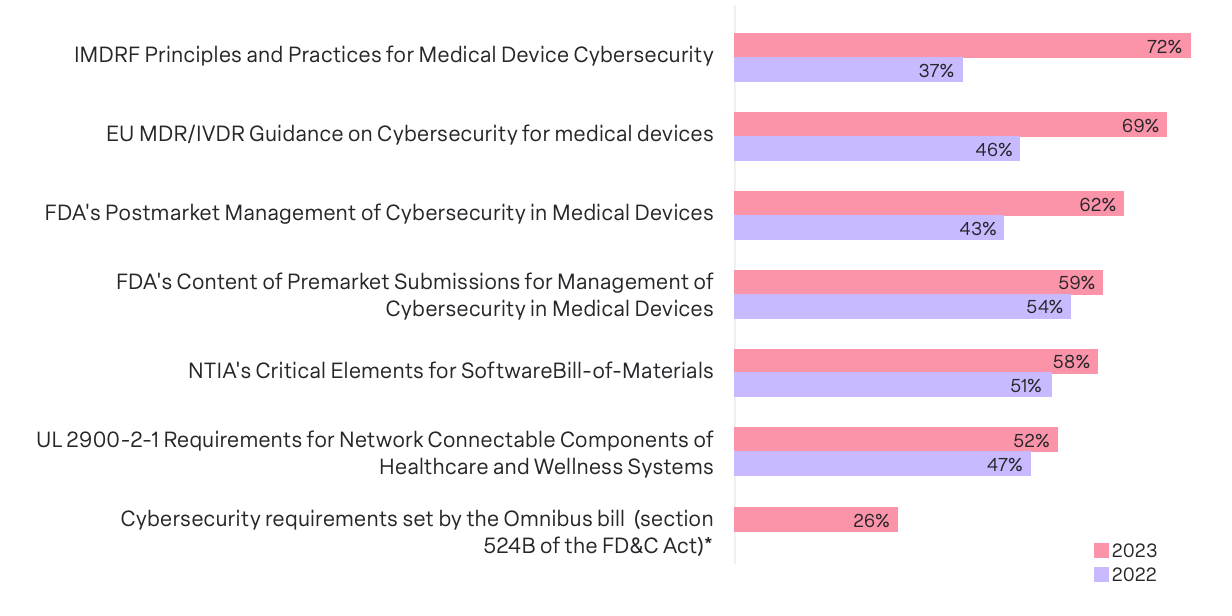
Our survey found that MDMs demonstrated heightened dedication to compliance with cyber regulations and guidelines in 2023 as compared with 2022.
The top guideline survey respondents report adhering to is the IMDRF Principles and Practices for Medical Device Cybersecurity, with an impressive compliance rate soaring from 37% in 2022 to a robust 72% in 2023. Similarly, compliance with the EU MDR/IVDR Guidance on Cybersecurity for medical devices saw a noteworthy ascent from 46% to 69%. Finally, compliance with the FDA’s Postmarket Management of Cybersecurity in Medical Devices regulation rates jumped from 43% to 62%.
These findings illuminate not just a surge in compliance efforts but also a strategic focus on specific regulations that wield considerable influence in shaping the industry’s security landscape. The collective industry response to these standards highlights a shared recognition of compliance as a paramount concern.
Maturity levels and compliance
An intriguing correlation comes to light when examining how MDMs’ medical device security program maturity levels affect their approach to compliance. Level 3 maturity companies emerge as frontrunners in the compliance realm, demonstrating a notable 73% adherence to the FDA’s Postmarket Cybersecurity Guidance. In stark contrast, their less mature counterparts, operating at lower maturity Levels 1 or 2, report a compliance rate of 56%.
This contrast underscores a compelling trend: as MDMs ascend the maturity ladder, their propensity to align with stringent regulatory standards, such as the FDA’s Postmarket Cybersecurity Guidance, significantly amplifies. This suggests that a mature security program not only fortifies devices against cyber threats but also positions MDMs to meet evolving compliance expectations with greater efficacy, fostering a holistic and resilient approach to medical device security.
Size matters
Size emerges as an additional factor influencing adherence to regulatory standards. Our survey reveals a nuanced interplay showcasing both strengths and weaknesses in compliance across different-sized companies. Larger companies, with their substantial resources, demonstrate higher compliance levels with the NTIA’s Critical Elements for Software Bill of Materials (SBOM) and the EU’s MDR/IVDR Guidance on Cybersecurity for medical devices.
However, a notable deviation is observed in compliance with the FDA’s Content of Premarket Submissions for Management of Cybersecurity in Medical Devices guidance. The survey found that larger companies lag in complying with the FDA’s Premarket guidance (47.6% compared to 63% in smaller companies). This suggests a potential discrepancy in prioritization or operational challenges specific to this regulatory aspect.
Regional dynamics
The landscape of compliance in the medical device industry exhibits distinctive regional dynamics, revealing significant variations in adherence to medical device regulatory standards. For U.S. companies, compliance with the FDA’s Premarket guidance, the FDA’s Postmarket guidance, and the Cybersecurity requirements set by the Omnibus bill (section 524B of the FD&C Act) stands at 81.6%, 81.6%, and 49%, respectively. In Germany and the rest of the world (ROW), the corresponding figures are around 50% for the two FDA guidances and approximately 14% for the Omnibus standard.
Compliance with the EU MDR/IVDR cybersecurity guidance is notably high in Germany (96%) and ROW (78%), contrasting with a lower rate of 39% in the U.S. Additionally, compliance levels for the IMDRF Principles and Practices for Medical Device Cybersecurity are at 77% in Germany and 81% for ROW, contrasting with 57% in the U.S. These differences align with expectations that regions tend to prioritize adherence to guidelines from their regulatory bodies.
Understanding and navigating the unique compliance expectations in different regions become paramount for global companies, as MDMs need to adopt a nuanced approach acknowledging the diverse regulatory landscapes they operate in.
Future commitments
As we delve into MDMs’ plans, a remarkable projection emerges with over 95% compliance anticipated by the end of 2023, across various regulations and standards. This proactive stance underscores the industry’s dedication to meeting evolving requirements and staying ahead of the curve in the ever-changing landscape of medical device security.
The projection of achieving such high compliance showcases a united effort among MDMs to navigate the intricate regulatory landscape effectively. This forward-looking approach is not only a testament to the industry’s dedication but also positions MDMs to meet and exceed evolving standards and regulations, ensuring the robust security of medical devices in the years to come.
Unveiling strategic insights in medical device security
As compliance takes center stage in the medical device security landscape, MDMs are not merely meeting standards; they are setting new benchmarks. The surge in compliance efforts reflects a commitment to excellence and a proactive response to legislative and regulatory pressures. It also does not come as a surprise, seeing that MDMs flagged compliance as a top priority in 2022, and 2023 saw much more legislative and regulatory pressure in the medical device space.
For a deeper dive into the nuanced findings, strategic insights, and comprehensive analysis, we invite you to download the full 2023 Medical Device Security Survey Report. Unlock a wealth of information to gain a clearer understanding of the intricate interplay between compliance, security, and the forward trajectory of medical device protection.
Download the full 2023 Medical Device Security Survey Report.