Over the last year, the FDA has released its final Premarket Authorization guidance– a document of cybersecurity guidelines that must be met to be sold. During the same year, the White House put out a holistic cybersecurity strategy and CISA came out with various security programs of its own.
What was missing was the overarching product security requirements that must be met by all federal agencies, ensuring the ultimate security is kept by both public and private institutions alike. Therefore, the US Government Accountability Office (GAO) put together a report with recommendations on how federal agencies, such as the FDA and CISA can better align their approach to product security.
The report, intended for congressional committees, is titled “Medical Device Cybersecurity: Health Care Providers Face Challenges in Securing Devices,” and sheds light on critical challenges and action items, such as companies accessing government support.
This blog aims to provide industry leaders with in-depth insights and practical strategies to navigate these challenges, emphasizing automation, vulnerability management, and the full product security lifecycle.
Overcoming Challenges in Accessing Federal Support
Health systems, providers, and patients often encounter obstacles in accessing federal support for medical device cybersecurity. This can be a result of not properly understanding the guidelines, lack of precedence, or other reasons. This gap can significantly impede the effective protection of sensitive health data and patient safety.
Practitioners can better understand their processes, identify where risks lie, and better communicate with oversight agencies by leveraging automation in the monitoring, reporting, and compliance processes. Beyond being useful as a communication tool, product security automation allows manufacturers to streamline the integration of federal cybersecurity guidelines into everyday practices. Examples are in monitoring regulatory changes, ensuring timely updates to security protocols, and maintaining comprehensive records for compliance purposes. More than anything, it can significantly reduce human errors while freeing up team members from conducting tedious repetitive tasks.

Enhancing Federal Agencies' Efforts in Cybersecurity
Federal agencies have been actively working to mitigate cybersecurity risks in medical devices. However, for product security practitioners, the key lies in understanding and adapting to these evolving efforts. Collaboration with agencies like the FDA, which they committed to in their RTA announcement, can provide valuable insights into emerging threats and best practices.
Industry leaders should invest in advanced vulnerability management systems that can detect and respond to new threats swiftly. These systems should be capable of identifying vulnerabilities while also providing actionable insights for quick remediation. Integrating these systems within the full product security lifecycle ensures that every stage, from design to post-market surveillance, is fortified against cyber threats.
Implementing recommendations for executive action
The GOA’s recommendations for executive action offer a roadmap for enhancing medical device cybersecurity. Product security practitioners should closely examine these recommendations, aligning their strategies accordingly. This involves adopting a holistic approach to cybersecurity, where protection measures are ingrained in every aspect of the product lifecycle, from conception to decommissioning.
Leaders should champion the implementation of these recommendations within their organizations, fostering a culture of cybersecurity awareness and preparedness. This includes regular training for staff, investment in cutting-edge security technologies, and rigorous testing and validation of security measures.
A peek into the future of federal product security
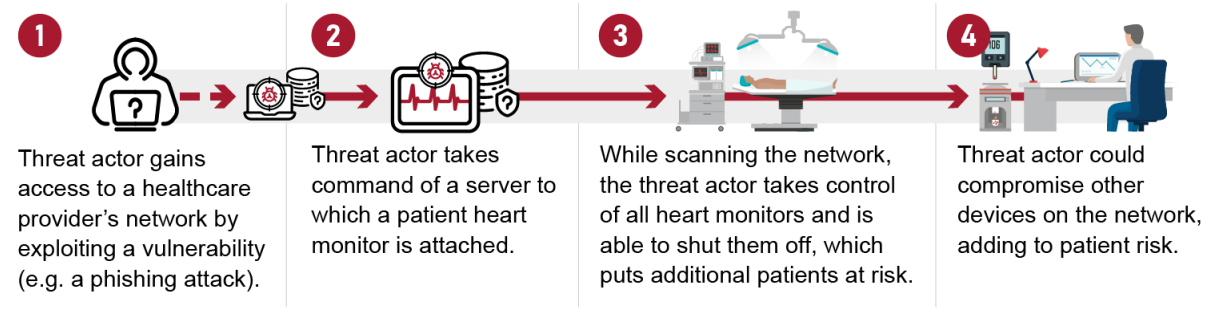
The GAO’s report highlights the extraordinary danger of a system being overtaken by threat actors and the risk to patient safety, should one of them penetrate the defenses of a medical device.
By focusing on automation, vulnerability management, and encompassing the entire product security lifecycle, practitioners can effectively address the highlighted challenges within their medical devices. Using automated workflows, organizations improve visibility across their product security processes so they can more easily identify challenges, reduce the strain on resources, avoid vulnerabilities, and better convey challenges across the medical device ecosystem.
As the medical device industry continues to grow and evolve, staying ahead with product security automation is not just a regulatory requirement but a commitment to patient safety and data protection.